Toei Shinyaku Obtains Patent for King Agaricus KA21 Intake-Induced Proliferation of Akkermansia muciniphila, Promising for Obesity Improvement, Diabetes Treatment, Cancer Immunotherapy, etc.
Application to Diet & Beauty Supplement Ingredients Expected.
東栄新薬株式会社
Toei Shinyaku Co., Ltd. (Location: Mitaka City, Tokyo; Representative Director: Akitomo Motoi) announces the successful outcome of collaborative research with the Immunology Department at Tokyo University of Pharmacy and Life Sciences. The research focused on our outdoor-cultivated King Agaricus KA21 strain (hereinafter referred to as Agaricus KA21), confirming its ability to promote the proliferation of Akkermansia muciniphila upon ingestion. In response to these findings, a patent registration has been completed for "Composition for promoting Akkermansia muciniphila proliferation applicable to medical and beauty purposes, and pharmaceuticals, food, and feed containing the same."
■ Overview
< Patent Number > Patent No. 7398714
< Patent Application Number > Application No. 2021-107016
< Submission Date > June 28, 2021 (Reiwa 3)
< Registration Date > December 7, 2023 (Reiwa 5)
< Patent Applicant > Toei Shinyaku Co., Ltd.

Patent certificate
Invention Title:
Composition for promoting Akkermansia muciniphila proliferation applicable to medical and beauty purposes, and pharmaceuticals, food, feed containing the same.
Technical Field:
The present invention relates to a composition for promoting the proliferation of Akkermansia muciniphila and its application in pharmaceuticals, food, and feed. Specifically, the pharmaceuticals are useful for improving obesity/metabolic syndrome, treating diabetes, alleviating intestinal inflammation, and enhancing cancer treatment for cancer immunotherapy. The food products are beneficial for improving obesity/metabolic syndrome, treating diabetes, alleviating intestinal inflammation, beauty purposes, and weight loss/diet.
About Akkermansia muciniphila:
Akkermansia muciniphila is a Gram-negative bacterium with a size of approximately 0.6-1.0 μm. It is a strict anaerobe that does not form spores and lacks motility. It is a commensal bacterium commonly present in the intestines of various mammals, including humans.
Recent studies have indicated a decrease in the abundance of Akkermansia muciniphila in the intestines of individuals with obesity or diabetes. Administration of Akkermansia muciniphila in mice has shown improvements in fat accumulation and insulin resistance, highlighting its potential relevance to conditions such as obesity. Additionally, Akkermansia muciniphila has been associated with anti-inflammatory effects in the intestines and has been suggested to enhance the efficacy of cancer immunotherapy.
About Agaricus blazei/brasiliensis/subrufescens:
Agaricus blazei is a type of mushroom widely used as a health food and supplement in the field of complementary and alternative medicine. Its characteristics and components vary depending on the strain, cultivation conditions, and origin. The National Institute of Health and Nutrition notes significant differences in quality among Agaricus-containing products.
Brazilian Outdoor-Cultivated Agaricus KA21 Strain (King Agaricus KA21):
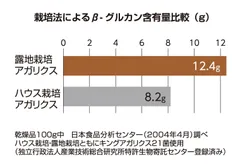
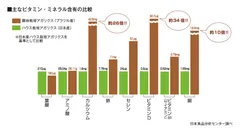
King Agaricus KA21 is cultivated outdoors in Brazil using the King Agaricus 21 (KA21) strain. Compared to conventional indoor cultivation, it grows larger in size and contains higher levels of key components such as β-glucans and vitamin D. Notably, it exhibits over five times the antioxidant activity.
Citations:
(*1) Nature Medicine 23,107-113,2017
(*2) Proc Natl Acad Sci USA 110,9066-9071(2013)
(*3) Gut.2011 Jan;60(1):34-40
(*4) Am J Gastroenterol.2010 Nov;105(11):2420-8
(*5) Science 05 Jan 2018:Vol. 359, Issue 6371, pp. 91-97
(*6) National Institute of Health and Nutrition, Material Information Databasehttps://www.nibiohn.go.jp/eiken/info/hf2.html
(*7) Japan Food Research Laboratories
(*8) IntJMedMushrooms.2019033173, pages 31-43

Outdoor Clutivated King Agaricus KA21

Left: Outdoor-cultivated, Right: Indoor-cultivated agaricus
Toei Shinyaku Co., Ltd.
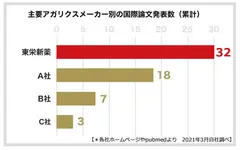
Toei Shinyaku Co., Ltd., the manufacturer of the KA21 strain, has dedicated over 27 years to the research and development of the KA21 strain. With an impressive track record, it has published a total of 32 international papers, showcasing the highest research output among Agaricus manufacturers (9). The company has collaborated on research projects with various institutions, including Azabu University School of Veterinary Medicine, Keio University SFC Research Institute, National Center for Geriatrics and Gerontology, Juntendo University School of Medicine, the University of Tokyo Food Safety Research Center, and the Immunology Department of Tokyo University of Pharmacy and Life Sciences. These collaborations have resulted in the presentation of diverse and valuable data on King Agaricus KA21.
(*9) Based on the number of international paper publications.
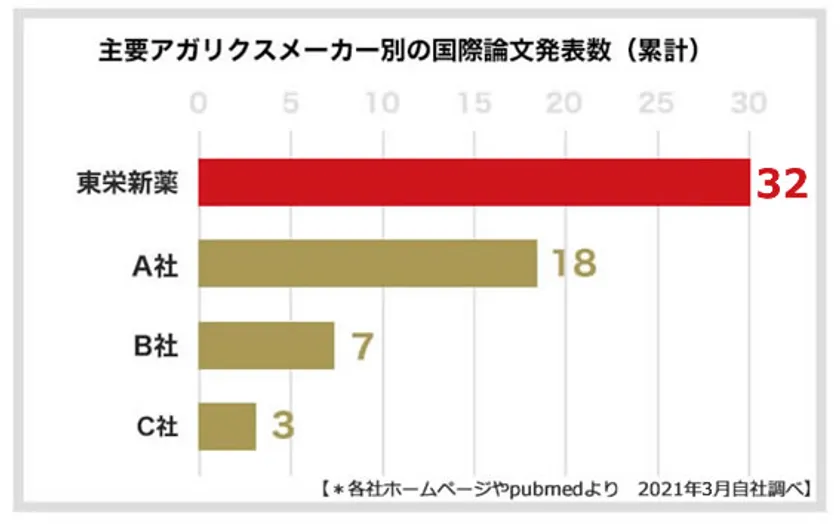
Number of Touei Shinyaku papers published
May 2021 from each company's website and pubmed (own research)
<Company Profile
Company name: Toei Shinyaku Co.Ltd.
Head Office: 1-11-23 Shimorenjaku, Mitaka-shi, Tokyo 181-0013, Japan
Representative: Akitomo Motoi, Representative Director
Capital :10 million yen
- Category:
- Corporate Trends
- Genres:
- General Business Medical care Beauty