Canine Cancer Test "Ark-Test" with a Little Blood ~The long-awaited "Cancer Risk Test" will start on November 1, 2024! Nobel Prize in Physiology or Medicine in 2024 The world's first(*1) cutting-edge testing technology using "microRNA The world's first(*1) cutting-edge testing technology based on "microRNA," which will be the focus of the Nobel Prize in Physiology or Medicine in 2024
株式会社メディカル・アーク
Medical Arc, Inc. (Location: Tama-Koganei Venture Port, Tokyo University of Agriculture and Technology, Representative Director: Hiroshi Ito, hereinafter referred to as "MA") has succeeded in detecting "microRNA" in blood exosomes specifically secreted by cancer cells and determining 12 cancer types with a small amount of blood. The company has successfully detected microRNAs in blood exosomes, which are specifically secreted by cancer cells, and started the commercialization of "Ark-Test".
Next, we established the "Ark-Test Cancer Risk Test" (hereinafter referred to as "Cancer Risk Test"), which tests only for the presence or absence of cancer, and will start its measurement from November 1, 2024.
(*1 Research conducted by MA)

Ark-Test" is expected to detect cancer in companion animals (pets) at an early stage.

The world's first testing technology to analyze "microRNA" in exosomes secreted by cancer cells
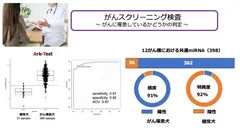
Over the past eight years, the world's first innovative testing technology has made further progress. Canine cancer (12 cancer types) can be identified with a small amount of blood. In addition, the "Cancer Risk Test," which tests only for the presence or absence of cancer, is now available at a low cost, with high sensitivity and high specificity.
Ark-Test commercialization footprint
January 11, 2023 Start of commercialization of 5 cancer types
((1) liver cancer, (2) oral melanoma, (3) urothelial carcinoma, (4) malignant lymphoma, and (5) mast cell tumor)
October 2, 2023 Commercialization of 8 cancer types started
(5 cancers + (6) hemangiosarcoma, (7) osteosarcoma, (8) squamous cell carcinoma)
January 11, 2024 Commercialization of 12 cancer types started (8 cancer types + (9) nasal adenocarcinoma, (10) breast cancer, (11) anal sac adenocarcinoma, (12) lung adenocarcinoma)
November 1, 2024 "Cancer Risk Screening" Commercialization Starts

Hiroshi Ito, Professor Emeritus, Tokyo University of Agriculture and Technology
Ark-Test" is the world's most advanced and innovative testing technology for companion animals (pets) that MA succeeded in commercializing earlier than humans, with the support of academia, including the world's No.1 researcher in exosome research (*2), Professor Takahiro Ochiya (Division of Molecular Cell Therapy, Center for Future Medical Research, Tokyo Medical University Institute of Medical Science). MA has succeeded in commercializing the world's most advanced and innovative testing technology for companion animals (pets) earlier than for humans.
(*2 https://www.tokyo-med.ac.jp/news/2024/0603_163021003409.html )

Professor Takahiro Ochiya, Tokyo Medical University
Detection of "microRNAs" commonly expressed in 12 cancer types!
This time, MA has succeeded not only in identifying the above 12 cancer types, but also in detecting "microRNAs" that are commonly expressed in all cancer types and detecting "cancer risk" only.
The "common microRNAs" have been confirmed to be expressed from other than the 12 cancer types, so it is highly likely that they are commonly expressed in a larger number of cancers.
The cost of the test is low, yet it has high sensitivity and specificity.
The "Cancer Risk Test" has a high sensitivity (91%) and specificity (92%), and detects only the presence or absence of cancer.
Furthermore, the cost of the test is as low as the cost of testing for one type of cancer (*3).
(*3 The cost of the test is not uniform because the test fee differs depending on the registered hospital.)

Serum is analyzed by digital PCR (high-performance testing equipment)
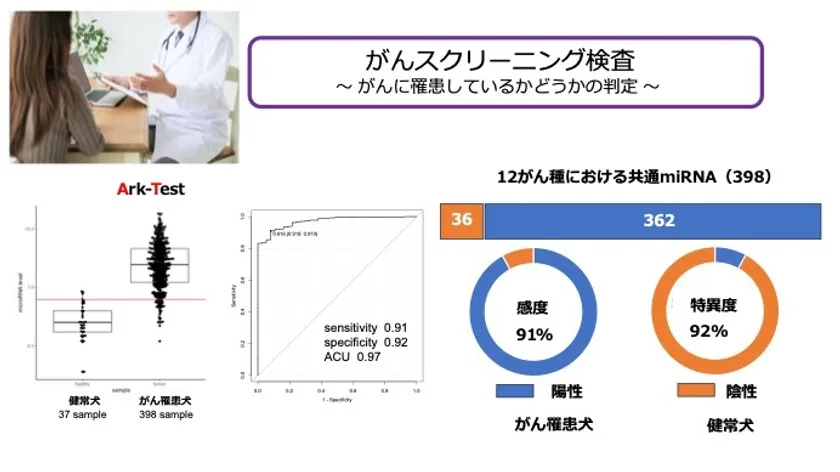
Cancer Risk Test" achieves high sensitivity (91%) and specificity (92%)
Expectations that the "Cancer Risk Test" will explosively save the lives of many pet dogs
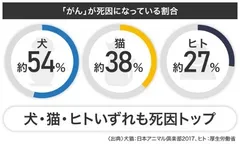
About 54% of canine deaths and 38% of feline deaths are caused by cancer.
Cancer is said to be curable for both humans and their pets if it is detected at an early stage, and is no longer an incurable disease under current medicine.
However, early detection of cancer in animals, which do not speak, is extremely difficult. Moreover, since cancer progresses at a rate five to seven times faster than in humans, it is not uncommon for the symptoms to be terminal when the disease is detected. If cancer screening were to be performed, CT and MRI diagnostic imaging under general anesthesia would be required, which is expensive and involves the risk of general anesthesia. Therefore, very few owners have ever performed cancer screening in a healthy state.
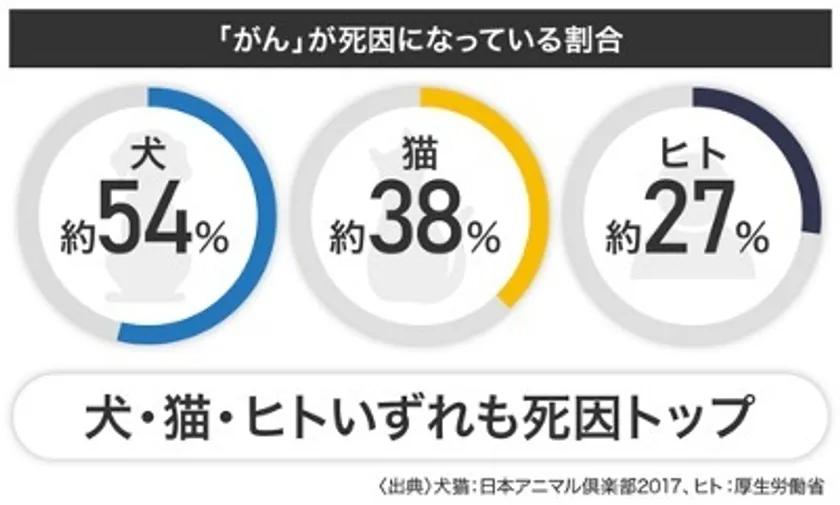
Cancer" is the cause of 54% of canine deaths.
With the advent of the "Ark-Test," it is now possible to determine the presence or absence of cancer, the identification of the cancer type, and even the stage of the cancer, using only a small amount of blood, with high sensitivity and specificity, and with the assurance of scientific evidence.
This "Cancer Risk Test" is expected to set the standard for "pet dog cancer screening" and has a high potential for early detection of cancer in many pet dogs and saving many lives.

MA, a company committed to "eradicating cancer in companion animals
■Partners in this project
Nomura Trading Co. President: Hideaki Fujiwara
Osaka Head Office: 1-7-3 Azuchi-machi, Chuo-ku, Osaka 541-8542, Japan
Tokyo Head Office: 4-3-13 Toranomon, Minato-ku, Tokyo 105-0001, Japan
https://www.nomuratrading.co.jp/
COREZON Corporation Representative Directors: Masaki Kobayashi / Retizo Kubo
Shinjuku Nomura Building 32F, 1-26-2 Nishi-Shinjuku, Shinjuku-ku, Tokyo 163-0532, Japan
Ace International Japan, Inc. Chairman: Shunsuke Miyao
Head Office: Ihara Takanawa Building 9F, 3-11-3 Takanawa, Minato-ku, Tokyo 108-0074, Japan
https://www.ace-international.com/
V-MAC Corporation President: Masashi Kato
4-8-10-1018 Tsukishima, Chuo-ku, Tokyo 104-0052, Japan
Information related to this business
MicroRNA Stabilizer Kit
Representative Director: Katsuyoshi Hara
7-13, Toyo 3-chome, Koto-ku, Tokyo 135-0016, Japan
Kits containing microRNA stabilizers necessary for testing can be purchased at "CYGNI VET".
https://vet.cygni.co.jp/disp/CSfGoodsPage_001.jsp?GOODS_NO=225841&c=9
Hospitals where the test can be performed
This test can be performed at designated veterinary hospitals nationwide (searchable from the URL below).
As of October 2024, about 670 hospitals https://search.medical-ark.com/hospital_search/
Free registration for designated hospitals
Veterinary professionals who wish to register as a newly designated hospital can do so free of charge at the following URL.
https://medical-ark.com/for_partners
□Member registration hospital benefits
Free start! Veterinary Hospital Digital Signage "PETDAYS
Designated hospitals can start the veterinary hospital digital signage "PETDAYS" provided by Chunichi Ad Planning Co. (No charge for production of hospital introduction videos, but monthly fees are charged separately.)
For inquiries about this inspection, please contact
Medical Arc, Inc.
2-24-16-103 Nakamachi, Koganei-shi, Tokyo 184-0012, Japan
Tama-Koganei Venture Port, Agriculture and Technology University
TEL : 042-316-6150
E-Mail: info@medical-ark.com
- Category:
- Corporate Trends
- Genres:
- Pets - Dogs Medical care Technology